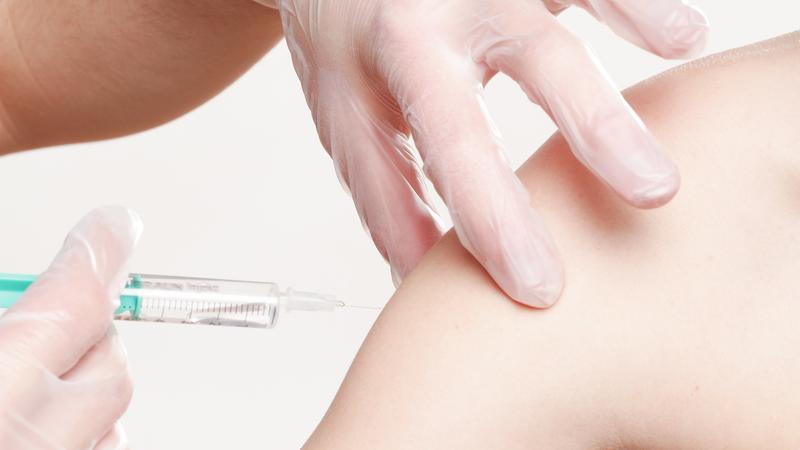
Health Canada gives its OK to Novavax’s COVID vaccine
Health Canada has approved a fifth COVID-19 vaccine for use in the country.
Novavax’s Nuvaxovid vaccine joins those from AstraZeneca, Janssen (Johnson & Johnson), Moderna and Pfizer on the list of approved COVID vaccines in Canada.
But Novavax’s offering is protein-based, making it the first of its kind to get approval in this country.
Experts say a non-mRNA vaccine could win over some vaccine-hesitant people who have still not received a dose of a COVID vaccine.



